Answer:
The new equilibrium concentration of HI: [HI] = 3.589 M
Step-by-step explanation:
Given: Initial concentrations at original equilibrium- [H₂] = 0.106 M; [I₂] = 0.022 M; [HI] = 1.29 M
Final concentrations at new equilibrium- [H₂] = 0.95 M; [I₂] = 0.019 M; [HI] = ? M
Given chemical reaction: H₂(g) + I₂(g) → 2 HI(g)
The equilibrium constant (
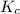 ) for the given chemical reaction, is given by the equation:
) for the given chemical reaction, is given by the equation:
![K_(c) = \frac {[HI]^(2)}{[H_(2)]\: [I_(2)]}](https://img.qammunity.org/2020/formulas/chemistry/college/tl4xlbsemf36y1mal7gzwhdvydsh25s6im.png)
At the original equilibrium state:
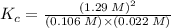
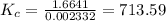
Therefore, at the new equilibrium state:
![K_(c) = \frac {[HI]^(2)}{(0.95\: M) * (0.019\: M)}](https://img.qammunity.org/2020/formulas/chemistry/college/98021lcxlxbospsfifh8r1qhdhxm9w7eur.png)
![\Rightarrow K_(c) = 713.59 = \frac {[HI]^(2)}{0.01805}](https://img.qammunity.org/2020/formulas/chemistry/college/2wpc7i8kwqeqew417fg8cvfudq90ijluhp.png)
![\Rightarrow [HI]^(2) = 713.59 * 0.01805 = 12.88](https://img.qammunity.org/2020/formulas/chemistry/college/1efq5olu1ubnldbzkannsf2mwplc9dwx19.png)
![\Rightarrow [HI] = \sqrt {12.88} = 3.589 M](https://img.qammunity.org/2020/formulas/chemistry/college/6c1yp2s89gzwifr2do7480uggejmophe6l.png)
Therefore, the new equilibrium concentration of HI: [HI] = 3.589 M