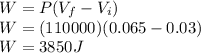Answer:
3850 J
Step-by-step explanation:
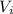 = initial volume of the gas = 30 L = 0.03 m³
= initial volume of the gas = 30 L = 0.03 m³
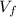 = final volume of the gas = 65 L = 0.065 m³
= final volume of the gas = 65 L = 0.065 m³
 = Constant pressure of the gas = 110 kPa = 110000 Pa
= Constant pressure of the gas = 110 kPa = 110000 Pa
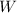 = Work done by the gas
= Work done by the gas
Since the pressure is constant, Work done by the gas is given as