Answer:
d. the atomic number remains the same.
Step-by-step explanation:
First at all it's important to know how to read nuclear information
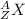
X is the atomic symbol, A the mass number and Z the atomic number of the element.
Gamma rays emitted on gamma decay are characterized as
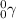
If we write the nuclear equation for the decay, we have that:
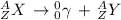
The sum of the mass numbers and atomic numbers on the right side has to be equal to the left side numbers of the equation, that means the mass number and the atomic number remains the same for the resulting atom to preserve the equality.