Answer:
177.993 MeV
Step-by-step explanation:
Nuclear fission refers to a process where a nucleus is broken into smaller ones.
m(Am) = 242.0595490 u, m(Sr) = 89.9077387 u, m(La) = 148.934733 u and m(n) = 1.008665 u. To find the mass of X, we sum up the masses on both side and subtract them according to the equation given
242 + X = 90 +149 + 4
X = 243 - 242 = 1
so X is a neutron
next we calculate the Δm ( change in mass)
Δm = mass reactant - mass of product
(242.0595490 + 1.008665 ) - ( 89.9077387 + 148.934733 + 4 (1.008665))
= 243.068214 - 242.8771317 = 0.1910823 u
using the formula
E = (Δm) c² = 0.1910823 u × c² ×
 ×
×
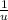 = 177.993 MeV
= 177.993 MeV