Answer:
B. +3 and +6
Step-by-step explanation:
The values of the Z
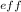 is estimated by subtracting the core electrons from the atomic number of the element.
is estimated by subtracting the core electrons from the atomic number of the element.
Oxygen has an atomic number of 8, 6 valence electrons, and 2 core electrons. Therefore the value of Z
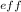 = 8-2 = +6
= 8-2 = +6
Boron has an atomic number of 5, 3 valence electrons and 2 core electrons. Therefore, the value of Z
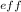 = 5-2 = +3
= 5-2 = +3