Answer:
The answer cannot be determined.
Step-by-step explanation:
The energy of the diver when he hits the pool will be equal to its potential energy
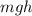 , and for the temperature of the pool to rise up, this energy has to be converted into the heat energy of the pool.
, and for the temperature of the pool to rise up, this energy has to be converted into the heat energy of the pool.
The change in temperature
 then will be
then will be
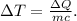
Where m is the mass of water in the pool, c is the specific heat capacity of water, and
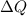 is the added heat which in this case is the energy of the diver.
is the added heat which in this case is the energy of the diver.
Since we do not know the mass of the water in the pool, we cannot make this calculation.