Answer:
c. −1.22 × 10^4 K
Step-by-step explanation:
Generally, the slope of an Arrhenius plot can be determined either from the graph of In k against 1/T or the available experimental data of the rate constant (k) and the absolute temperature. Mathematically,
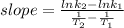
Where:
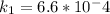 L/(mol.s)
L/(mol.s)
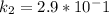 L/(mol.s)
L/(mol.s)
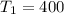 K
K
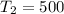 K
K
slope = (ln 2.9*10^-1 - ln 6.6*10^-4)/(1/500 - 1/400)
slope = (-1.238+7.323)/(0.002-0.0025)
slope = 6.085/-0.0005 = -1.22*10^4 K