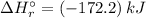Answer:
The Standard enthalpy of reaction:
Step-by-step explanation:
Given- Standard Heat of Formation:
![\Delta H_(f)^(\circ ) [H_(3)AsO_(4)(aq)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/v5efqb9qh80q08scpf8qpb464zfm77posc.png) = -904.6 kJ/mol
= -904.6 kJ/mol
![\Delta H_(f)^(\circ ) [H_(2)(g)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/81kh53si2d99f2djltj1ha85dk269d6xe2.png) = 0 kJ/mol,
= 0 kJ/mol,
![\Delta H_(f)^(\circ ) [AsH_(3)(g)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/j8jf4ffc28z4ef9eb0fcc1jqi2lm84xpgc.png) = +66.4 kJ/mol
= +66.4 kJ/mol
![\Delta H_(f)^(\circ ) [H_(2)O(l)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/hp33vty2ez21jeols9kzuiomwyh4o3q3ls.png) = -285.8 kJ/mol
= -285.8 kJ/mol
Given chemical reaction: H₃AsO₄(aq) + 4H₂(g) → AsH₃(g) + 4H₂O(l)
The standard enthalpy of reaction:
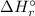 = ?
= ?
To calculate the Standard enthalpy of reaction (
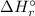 ), we use the equation:
), we use the equation:
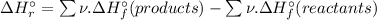
![\Delta H_(r)^(\circ ) = [1 * \Delta H_(f)^(\circ ) [AsH_(3) (g)] + 4 * \Delta H_(f)^(\circ ) [H_(2)O(l)]] - [1 * \Delta H_(f)^(\circ ) [H_(3)AsO_(4)(aq)] + 4 * \Delta H_(f)^(\circ ) [H_(2)(g)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/4c7sgmjtv1ad1gghezyz22jbnwhvo14jte.png)
![\Rightarrow \Delta H_(r)^(\circ ) = [1 * (+66.4\,kJ/mol) + 4 * (-285.8\,kJ/mol) ] - [1 * (-904.6\,kJ/mol) + 4 * (0\,kJ/mol)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/cv6zpg164n4p4adnv53t5eojyoe6ag3g6q.png)
![\Rightarrow \Delta H_(r)^(\circ ) = [-1076.8\, kJ] - [-904.6\,kJ]](https://img.qammunity.org/2020/formulas/chemistry/high-school/cq0gzbo5n33aslbsbb9vbixbppklxe5kyu.png)
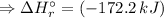
Therefore, the Standard enthalpy of reaction: