Answer:
Option (B) is correct
Step-by-step explanation:
Oxidation:
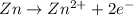
Reduction:
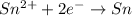
---------------------------------------------------------------------------
Overall:
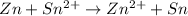
Nernst equation for this cell reaction at
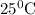 :
:
![E_(cell)=(E_(Sn^(2+)\mid Sn)^(0)-E_(Zn^(2+)\mid Zn)^(0))-(0.059)/(n)log([Zn^(2+)])/([Sn^(2+)])](https://img.qammunity.org/2020/formulas/chemistry/college/aixc1q174fvwk3qhd1iw695at5v4tcnxrs.png)
Where, n is number of electron exchanged and species inside third bracket represents concentrations in molarity.
Here, n = 2,
 and
and
![[Zn^(2+)]=2.5* 10^(-3)M](https://img.qammunity.org/2020/formulas/chemistry/college/xinnpiq7sjnb8hvkqvw97rng95khc29uhp.png)
So, plug in all the given values into above equation:
![0.660V=(-0.136V+0.76V)-(0.059)/(2)log(2.5* 10^(-3)M)/([Sn^(2+)])](https://img.qammunity.org/2020/formulas/chemistry/college/65ou7mlt7qsvq6tbh7655qyn1ognzrf6gb.png)
So,
![[Sn^(2+)]=4.2* 10^(-2)M](https://img.qammunity.org/2020/formulas/chemistry/college/vbr55h2qsdfnfva7vms961vx5og3urnv7v.png)
As the value "0.059" varies from literature to literature and
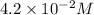 is most closest to
is most closest to
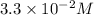 therefore option (B) is correct.
therefore option (B) is correct.