Answer:
Rate of respiration will be higher at 22 degree Celsius as compared to rate of respiration at 10 degree Celsius.
Step-by-step explanation:
As per the ideal gas law (Boyle's law)
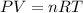
Where P stands for pressure, V stands for volume, n stands for number of moles, R stands for gas constant and T stands for temperature. As we can see in this equation that if pressure remains constant then the temperature is directly proportional to the volume of gas. Thus, as the temperature increases the rate of respiration will also increase.
Hence, the rate of respiration will be higher at 22 degree Celsius as compared to rate of respiration at 10 degree Celsius.