A) 0.10 m (NH₄)₂SO₄ has the greatest freezing-point depression
Further explanation
Given
The aqueous solutions
Required
The greatest freezing-point depression
Solution
Colligative freezing point
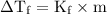
for electrolyte :
ΔTf = Kf x m x i
i (van't Hoff factor ) = 1 + (n-1) α
All solutions in the problem have a molal concentration (0.1 m) and the same solvent ⇒ assuming water (The same Kf) so that what affects the value of ΔTf is the value of i (For methanol which is not an electrolyte, we put it aside )
Assuming the degree of electrolyte ionization α= 1, the magnitude i is determined by the number of ions produced by the electrolyte (n)
(NH₄)₂SO₄ ⇒ 2NH₄⁺ + SO₄²⁻ : 3 ions
MnSO₄ ⇒ Mn²⁺ + SO₄²⁻ : 2 ions
NaF ⇒ Na⁺ + F⁻ : 2 ions
KCl ⇒ K⁺ + Cl⁻ : 2 ions