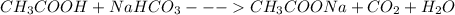Answer:
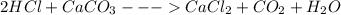
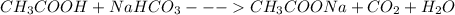
Step-by-step explanation:
Hydrocholoric acid is written as HCl
Calcium carbonate is written as
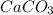
For a balanced equation, 2 moles of Hydrochloric acid reacts with 1 mole of calcium carbonate to give one mole of calcium chloride, 1 mole of carbon dioxide and 1 mole of water respectively.
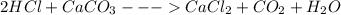
Baking soda is written as
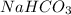
Vinegar is written as
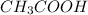
1 mole of vinegar reacts with 1 mole of baking soda to give 1 mole of sodium acetate, one mole of carbon dioxide and 1 mole of water respectively.