Answer:

Step-by-step explanation:
To convert from moles to grams, the molar mass must be used.
1. Find Molar Mass
The compound is sodium sulfide: Na₂S
First, find the molar masses of the individual elements in the compound: sodium (Na) and sulfur (S).
- Na: 22.9897693 g/mol
- S: 32.07 g/mol
There are 2 atoms of sodium, denoted by the subscript after Na. Multiply the molar mass of sodium by 2 and add sulfur's molar mass.
- Na₂S: 2(22.9897693 g/mol)+(32.07 g/mol)=78.0495386 g/mol
This number tells us the grams of sodium sulfide in 1 mole.
2. Calculate Grams
Use the number as a ratio.
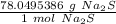
Multiply by the given number of moles, 3.46.
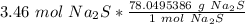
The moles of sodium sulfide will cancel.
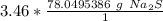
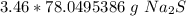
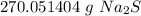
3. Round
The original measurement of grams, 3.46, has 3 significant figures. We must round our answer to 3 sig figs.
For the answer we calculated, that is the ones place.
The 0 in the tenth place tells us to leave the 0 in the ones place.
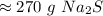
There are about 270 grams of sodium sulfide in 3.46 moles.