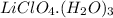Answer:
The formula is =
Step-by-step explanation:
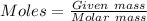
% of Li = 4.330
Molar mass of Li = 6.941 g/mol
% moles of Li =
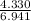 = 0.62382
= 0.62382
% of Cl = 22.10
Molar mass of Cl = 1.00784 g/mol
% moles of Cl =
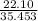 = 0.62336
= 0.62336
% of O = 39.89
Molar mass of O = 15.999 g/mol
% moles of O =
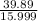 = 2.49328
= 2.49328
% of H₂O = 33.69
Molar mass of H₂O = 18.01528 g/mol
% moles of H₂O =
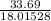 = 1.87007
= 1.87007
Taking the simplest ratio for Li , Cl, O, and H₂O as:
0.62382 : 0.62336 : 2.49328 : 1.87007
= 1 : 1 : 4 : 3
The formula is =