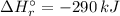Answer:
The Standard enthalpy of reaction:
Step-by-step explanation:
Given- Standard Heat of Formation:
![\Delta H_(f)^(\circ ) [P_(4)O_(10)(s)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ym9t3kpievj2jr3kyxkxl4kry1j0gcgmjz.png) = -3110 kJ/mol,
= -3110 kJ/mol,
![\Delta H_(f)^(\circ ) [H_(2)O(l)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/hp33vty2ez21jeols9kzuiomwyh4o3q3ls.png) = -286 kJ/mol,
= -286 kJ/mol,
![\Delta H_(f)^(\circ ) [H_(3)PO_(4)(s)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/c4obpuh0m7128w15s4wntrqkskm6uefh9x.png) = -1279 kJ/mol
= -1279 kJ/mol
Given chemical reaction: P₄O₁₀(s) + 6H₂O → 4H₃PO₄
The standard enthalpy of reaction:
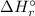 = ?
= ?
To calculate the Standard enthalpy of reaction (
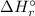 ), we use the equation:
), we use the equation:
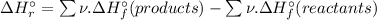
![\Delta H_(r)^(\circ ) = [4 * \Delta H_(f)^(\circ ) [H_(3)PO_(4)(s)]] - [1 * \Delta H_(f)^(\circ ) [P_(4)O_(10)(s)] + 6 * \Delta H_(f)^(\circ ) [H_(2)O(l)]]](https://img.qammunity.org/2020/formulas/chemistry/high-school/zblkjryvzn41kdgukocghll4km82pstslh.png)
![\Rightarrow \Delta H_(r)^(\circ ) = [4 * (-1279\, kJ/mol)] - [1 * (-3110\, kJ/mol) + 6 * (-286\, kJ/mol)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ku26qeh6s30jesfh7fifuu8izhhrsiuqo5.png)
![\Rightarrow \Delta H_(r)^(\circ ) = [-5116\, kJ] - [-3110\, kJ -1716\, kJ]](https://img.qammunity.org/2020/formulas/chemistry/high-school/e5xqdbf5wqajbk1jjk42vcxoerqr5j2d8n.png)
![\Rightarrow \Delta H_(r)^(\circ ) = [-5116\, kJ] - [-4826\, kJ] = -290\,kJ](https://img.qammunity.org/2020/formulas/chemistry/high-school/9rxl67ia3zmadtgrvhi7cfjo46bk356piq.png)
Therefore, the Standard enthalpy of reaction: