Answer:
Actual yield of
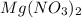 is 344.3 g
is 344.3 g
Step-by-step explanation:
According to balanced equation, 1 mol of Mg produces 1 mol of
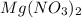
Molar mass of Mg = 24.305 g/mol and molar mass of
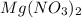 = 148.3 g/mol
= 148.3 g/mol
So, 24.305 of Mg produces 148.3 g of
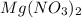
Hence, 119.3 of Mg produces
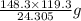 of
of
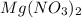 or 727.9 g of
or 727.9 g of
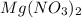
Hence, theoretical yield of
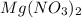 = 727.9 g
= 727.9 g
So, actual yield of
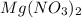 =
=
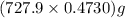 = 344.3 g
= 344.3 g