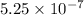The question is incomplete, here is the complete question.
An aqueous solution at 25°C has a
 concentration of
concentration of
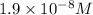 . Calculate the
. Calculate the
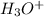 concentration. Be sure your answer has the correct number of significant digits.
concentration. Be sure your answer has the correct number of significant digits.
Answer: The hydronium ion concentration of the solution is
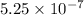
Step-by-step explanation:
To calculate pOH of the solution, we use the equation:
![pOH=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/n477c3o3xy8p6ug3ipfjqh9fd53hdrrjyq.png)
We are given:
![[OH^-]=1.9* 10^(-8)M](https://img.qammunity.org/2020/formulas/chemistry/college/ntqehcmir7a84ydi7v2zoy2hare0ardg3q.png)
Putting values in above equation, we get:
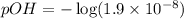
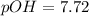
To calculate the hydronium ion concentration, we first calculate pH of the solution, which is:
pH + pOH = 14
pH + 7.72 = 14
pH = 14 - 7.72 = 6.28
To calculate the hydronium ion concentration of the solution, we use the equation:
![pH=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/f390cegazdnm7uy3e4lyqajx4gquacwg62.png)
We are given:
pH = 6.28
Putting values in above equation, we get:
![6.28=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/college/bawdpuu68tkz27y0vp3zls8a7ardcauz4h.png)
![[H_3O^+]=5.25* 10^(-7)](https://img.qammunity.org/2020/formulas/chemistry/college/qwtkhmeq0u5ckdui3mqje3q347pdqa0lz7.png)
Hence, the hydronium ion concentration of the solution is