Answer:
Specific rotation of mixture = D. 0.84 °
Step-by-step explanation:
Enantiomeric excess = % of one enantiomer - % of the other enantiomer = 75-25 % = 50 %
Also,
Enantiomeric excess =
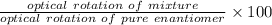
Optical rotation of pure enantiomer = 1.68 °
Applying the values as:-
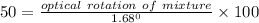
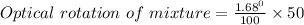
Specific rotation of mixture = 0.84 °