Answer:
(a) [A] = 0.13 M, [B]= 0.23 M and [C] = 0.17 M.
(b) Option B.
Step-by-step explanation:
The reaction given:
A(aq) + B(aq) ⇄ C(aq) (1)
Initial: 0.30M 0.40M 0M (2)
Equilibrium: 0.3 - x 0.4 - x x (3)
The equation of Gibbs free energy of the reaction (1) is the following:
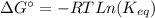 (4)
(4)
where ΔG°: is the Gibbs free energy change at standard conditions, R: is the gas constant, T: is the temperature and
 : is the equilibrium constant
: is the equilibrium constant
(a) To calculate the concentrations of A, B, and C at equilibrium, we need first determinate the equilibrium constant using equation (4), with ΔG°=-4.51x10³J/mol, T=25 + 273 = 298 K, R=8.314 J/K.mol:
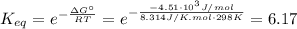 (5)
(5)
Now, we can calculate the concentrations of A, B, and C at equilibrium using the equilibrium constant calculated (5):
![K_(eq) = ([C])/([A][B]) = (x)/((0.3 - x)(0.4 - x))](https://img.qammunity.org/2020/formulas/chemistry/high-school/8kf6g81zq90vwurl2j8m6aqxv244ylumck.png) (6)
(6)
Solving equation (6) for x, we have two solutions x₁=0.69 and x₂=0.17, and by introducing the solution x₂ into equation (3) we can get the concentrations of A, B, and C at equilibrium:
![[A] = 0.3 - x_(2) = 0.3 - 0.17 = 0.13 M](https://img.qammunity.org/2020/formulas/chemistry/high-school/cjj0yepozkasac4431wl8e1a96nlasozpe.png)
![[B] = 0.4 - x_(2) = 0.4 - 0.17 = 0.23 M](https://img.qammunity.org/2020/formulas/chemistry/high-school/d0ns6xiq9jl58g8m0qov7fkgxoz2kz1n6t.png)
![[C] = x = 0.17 M](https://img.qammunity.org/2020/formulas/chemistry/high-school/on9fw9id3as0mz1ooeg8g8tby127b1kp13.png)
Notice that the solution x₁=0.69 would have given negative values of the A and B concentrations.
(b) If the reaction had a standard free-energy change of +4.51x10³J/mol, the equilibrium constant would be:
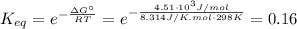
By solving the equation (6) for x, with the equilibrium constant calculated, we can get again two solutions x₁ = 6.9 and x₂= 0.017, and by introducing the solution x₂ into equation (3) we can get the concentrations of A, B, and C at equilibrium:
![[A] = 0.3 - x_(2) = 0.3 - 0.017 = 0.28 M](https://img.qammunity.org/2020/formulas/chemistry/high-school/z72d9qqccoqw31lety7q7cjb0md329p505.png)
![[B] = 0.4 - x_(2) = 0.4 - 0.017 = 0.38 M](https://img.qammunity.org/2020/formulas/chemistry/high-school/mztbifmmed7ckbdch9no75wudi0endhf9i.png)
![[C] = x = 0.017 M](https://img.qammunity.org/2020/formulas/chemistry/high-school/fymn5hs29ayts1ynxonvn6b8uzdb6tf7ko.png)
Again, the solution x₁=6.9 would have given negative values of the A and B concentrations.
Hence, the correct answer is option B: there would be more A and B but less C.
I hope it helps you!