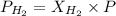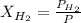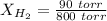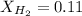Answer:
d. 0.11
Step-by-step explanation:
According to the Dalton's law of partial pressure, the total pressure of the gaseous mixture is equal to the sum of the pressure of the individual gases. Also, the partial pressure of the gas is equal to the product of the mole fraction and total pressure.
Total pressure, P = 800 torr
Partial pressure of hydrogen gas = 90 torr
So,