Answer:
Concentration of
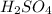 is 0.675 M
is 0.675 M
Step-by-step explanation:
According to balanced equation, 2 moles of KOH neutralizes 1 mol of
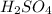 .
.
18.90 mL of 2.50M KOH =
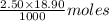 of KOH = 0.04725 moles of KOH
of KOH = 0.04725 moles of KOH
If molarity of
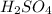 is C (M) then-
is C (M) then-
moles of
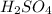 are neutralized =
are neutralized =
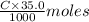 of
of
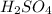
Hence,
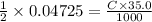
or, C = 0.675
So, concentration of
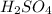 is 0.675 M
is 0.675 M