Answer:
Final volume = 0.42 cubic meters
Step-by-step explanation:
The question is incomplete. The initial conditions are
Initial temperature ,
 = 310 K
= 310 K
Initial volume ,
 = 0.5 cubic meter
= 0.5 cubic meter
Number of moles = 2.5
Since, from the ideal gas equation we get,
PV = nRT
P
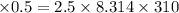
 = 12886.7 Pa
= 12886.7 Pa
Final pressure= 15464.04 Pa
Since temperature is constant,
PV = constant
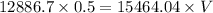
V = 0.42 cubic meters.
Hence final volume = 0.42 cubic meters