Answer:
A) He because the He atoms are moving at a higher average speed than the Ne atoms.
Step-by-step explanation:
The expression for the mean speed is:
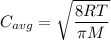
R is Gas constant having value = 8.314 J / K mol
M is the molar mass of gas
T is the temperature
Thus, as seen from the formula,
The average speed of the gas is inversely proportional to the square root of the molar mass of the gas
So, Helium has lower molecular mass than neon and thus, The speed of the sound in helium is greater when compared to that of neon.
Thus, He will escape faster through the pinhole.
Option A. is correct.