Answer:
e. 0.0822 M
Step-by-step explanation:
Considering:-
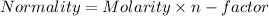
So, Given that:-
Molarity of
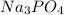 = 1.28 M
= 1.28 M
n-factor of
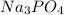 = 3
= 3
So,
Normality of
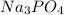 = 3*1.28 N = 3.84 N
= 3*1.28 N = 3.84 N
Considering:-
At equivalence point
Gram equivalents of
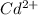 = Gram equivalents of
= Gram equivalents of
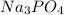
So,
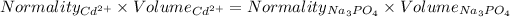
Given that:
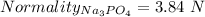
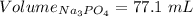
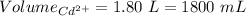
So,
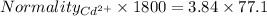
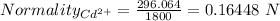
Also,
n-factor of
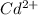 = 2
= 2
So,
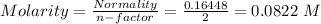
Hence, e is the answer.