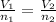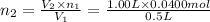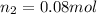Step-by-step explanation:
1) PV = nRT(ideal gas equation )
All of the gas laws equations that relate to the ideal gas law:
1. Boyle's law. This law states that pressure is directly proportional to the volume of the gas at constant temperature.
The equation given by this law is:
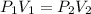
where,
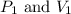 are initial pressure and volume.
are initial pressure and volume.
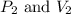 are final pressure and volume.
are final pressure and volume.
2. Charles' Law. This law states that volume of the gas is directly proportional to the temperature of the gas at constant pressure.
Mathematically,
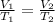
where,
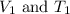 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.
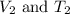 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.
3. Gay-Lussac Law. This law states that pressure of the gas is directly proportional to the temperature of the gas at constant pressure.
Mathematically,
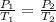
where,
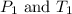 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.
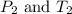 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.
4. Avogadro's law. This law states that volume is directly proportional to number of moles at constant temperature and pressure.
The equation used to calculate number of moles is given by:
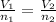
where,
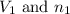 are the initial volume and number of moles
are the initial volume and number of moles
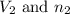 are the final volume and number of moles
are the final volume and number of moles
2)
Initial moles of gas =
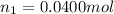
Initial volume of the gas =
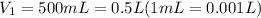
Final moles of gas =
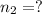
Final volume of the gas =

Equation used to find the amount of gas added is Avogadro's law equation :