Answer:
-1.78 V
Step-by-step explanation:
There are several rules required to calculate the cell potential:
- given standard cell potential, we may reverse the equation: the products of a given reaction become our reactants, while reactants become our products in the reversed equation. For a reversed equation, we change the sign of the cell potential to the opposite sign;
- if we multiply the whole equation by some number, this doesn't influence the cell potential value. It only produces a different expression in the equilibrium constant.
That said, notice that the initial reaction with respect to the final reaction is:
- reversed: chromium(III) cation and chloride anion become our reactants as opposed to the products in the initial reaction, so we change the sign of the cell potential to a negative value of -1.78 V;
- each coefficient is multiplied by a fraction of
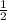 . It doesn't influence the value of the cell potential.
. It doesn't influence the value of the cell potential.
Thus, we have a cell of E = -1.78 V.