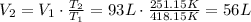Answer:
56 L
Step-by-step explanation:
We're dealing with a gas in this problem. We may, therefore, apply the ideal gas law for this problem:
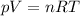
We now that we have a constant pressure. Besides, R, the ideal gas law constant, is also a constant number. Let's rearrange the equation so that we have all constant variables on the right and all changing variables on the left:
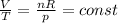
This means the ratio between volume and temperature is a constant number. For two conditions:
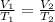
Given initial volume of:
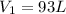
Convert the initial temperature into Kelvin:
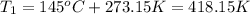
Convert the final temperature into Kelvin:
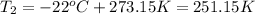
Rearrange the equation for the final volume: