Answer:
The pH of 0.1 M BH⁺ClO₄⁻ solution is 5.44
Step-by-step explanation:
Given: The base dissociation constant:
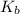 = 1 × 10⁻⁴, Concentration of salt: BH⁺ClO₄⁻ = 0.1 M
= 1 × 10⁻⁴, Concentration of salt: BH⁺ClO₄⁻ = 0.1 M
Also, water dissociation constant:
 = 1 × 10⁻¹⁴
= 1 × 10⁻¹⁴
The acid dissociation constant (
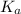 ) for the weak acid (BH⁺) can be calculated by the equation:
) for the weak acid (BH⁺) can be calculated by the equation:
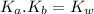
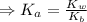
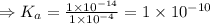
Now, the acid dissociation reaction for the weak acid (BH⁺) and the initial concentration and concentration at equilibrium is given as:
Reaction involved: BH⁺ + H₂O ⇌ B + H₃O+
Initial: 0.1 M x x
Change: -x +x +x
Equilibrium: 0.1 - x x x
The acid dissociation constant:
![K_(a) = (\left [B \right ] \left [H_(3)O^(+)\right ])/(\left [BH^(+) \right ]) = ((x)(x))/((0.1 - x)) = (x^(2))/(0.1 - x)](https://img.qammunity.org/2020/formulas/chemistry/high-school/6noo7fsadyhq0ybes3mbje8xmyyqgpajru.png)
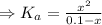
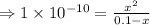


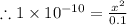
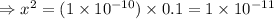
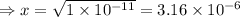
Therefore, the concentration of hydrogen ion: x = 3.6 × 10⁻⁶ M
Now, pH = - ㏒ [H⁺] = - ㏒ (3.6 × 10⁻⁶ M) = 5.44
Therefore, the pH of 0.1 M BH⁺ClO₄⁻ solution is 5.44