Answer:
2.53 grams of hydrogen gas will be produced and 12.2 many grams of the excess reactant i.e. calcium will be left over.
Step-by-step explanation:
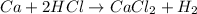
Moles of calcium =
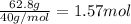
Moles of HCl =
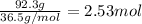
According to reaction, 2 moles of HCl reacts with 1 mole of calcium :
Then 2.53 moles of HCl will recat with :
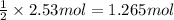 of calcium.
of calcium.
As we can see moles of calcium are in excessive amount. Hence calcium is an excessive reagent.
Moles of calcium left unreacted =1.57 mol - 1.265 mol =0.305 mol
Mass calcium left unreacted = 0.305 mol × 40 g/mol =12.2 g
Since, calcium is an excessive reagent HCl is limiting reagent and the amount of hydrogen gas produced will depend on HCl .
According to reaction, 2 moles of HCl gives 1 mole of hydrogen gas.
Then 2.53 moles of HCl will give:
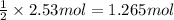 of hydrogen gas.
of hydrogen gas.
Mass of 1.265 mol of hydrogen gas = 1.265 mol × 2 g/mol = 2.53 g
2.53 grams of hydrogen gas will be produced and 12.2 many grams of the excess reactant i.e. calcium will be left over.