Step-by-step explanation:
The given data is as follows.
V = 0.389 L = 0.389 kg
m = 389 g of water (as 1 kg = 1000 g)
wavelength = 12.9 cm
dT =
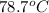
First, we will identify the heat absorbed as follows.
Q =
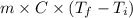
Putting the given values into the above formula as follows.
Q =
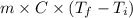
Q =
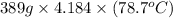
= 128090.231 J
Now, as we know that
Energy (E) =
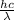
or,
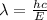
where, h = Planck's Constant =
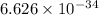 J s
J s
c = speed of particle (i.e. light) =
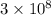 m/s
m/s
E = energy per particle J/photon
Hence, calculate the energy as follows.
E =
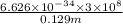
(As 1 m = 100 cm)
=
 J/photons
J/photons
Hence, number of photons present will be calculated as follows.
n photons =
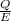
=
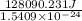
=
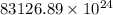
=
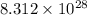
Thus, we can conclude that number of photons released are
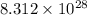 .
.