We will start by finding the heat in state one through the thermal efficiency or efficiency of a thermal machine is a coefficient or dimensionless ratio calculated as the ratio of the energy produced (in an operating cycle) and the energy supplied to the machine. Mathematically it is the relationship between the work generated and the heat emanated.
So,
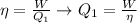
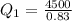
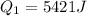
The total change of energy is equivalent to 4500J and this is equal by conservation of energy to the total change in heat. So:
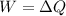
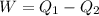
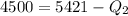
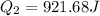
Therefore the energy which is discharged to the lower temperature reservoir every cicle is 921.7J