Answer:
1 ring
Step-by-step explanation:
The molecular formula of a saturated alkane with 12 carbon atoms is
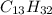 while that of a cyclic alkane with 12 carbon atoms is
while that of a cyclic alkane with 12 carbon atoms is
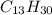 . Thus, if six hydrogen atoms are removed from the compound, this would translate into a three double bonds. Therefore, the number of rings in the original compound would be one (1).
. Thus, if six hydrogen atoms are removed from the compound, this would translate into a three double bonds. Therefore, the number of rings in the original compound would be one (1).