Answer:
1.38*10^4 L or 0.138*10^2 m^3
Step-by-step explanation:
The balanced equation for the reaction is:
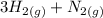 ⇒
⇒
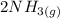
In the chemical equation above, 3 moles of hydrogen gas react with 1 mole of nitrogen gas to produce 2 moles of ammonia gas. A STP, 1 mole of gas is equivalent to 22.4 L. Therefore,
6.9 m^3 of nitrogen gas will be equivalent to 6900 L. In addition, 6900L will be equivalent to 6900/22.4 = 308.036 moles
1 mole of nitrogen produced 2 moles of ammonia gas, therefore, 308.04 moles of nitrogen gas would produce 2*308.036 moles = 616.07 moles of ammonia gas
At STP, 1 mole of gas is equivalent to 22.4 L, thus 616.07 moles will be equivalent to 22.4*616.07 = 13800 L or 13.8 m^3