Answer:
Therefore, the temperature of the water after 96 min (5760 s) = 279.25 K = 6.25 ℃
Step-by-step explanation:
Given: Time: t₁ = 24 min = 24 × 60 s = 1440 s
Time: t₂ = 96 min = 96 × 60 s = 5760 s (∵ 1 min = 60 s)
Initial Temperature of the water: T(0) = 100℃ = 100 + 273 K = 373 K
Ambient temperature (temp of the freezer): Tₐ = 0℃ = 0 + 273 K = 273 K
Temperature of the water at time t₁: T(t₁) = 50℃ = 50 + 273 K = 323 K
(∵ 0℃ = 273 K)
Temperature of the water at time t₂: T(t₂) = ? K
According to the Newton's Law of Cooling:
![[T(t) - T_(a)] = [T(0) - T_(a)] e^(-kt)](https://img.qammunity.org/2020/formulas/chemistry/high-school/lkw5cbg2eoftnpta5wjv8kqz320gh9bqi4.png)
Here, k is the cooling constant
![\therefore [T(t_(1)) - T_(a)] = [T(0) - T_(a)] e^{-kt_(1)}](https://img.qammunity.org/2020/formulas/chemistry/high-school/uczrx92xckhhb79esw5irc0lu4lyivskds.png)
![\Rightarrow [323\, K - 273\, K] = [373\, K - 273\, K] e^(-k(1440 sec))](https://img.qammunity.org/2020/formulas/chemistry/high-school/d1z5h92n9t8j8vvqa9b5nd0bv733jhjsl5.png)
![\Rightarrow [50] = [100] e^(-k(1440 sec))](https://img.qammunity.org/2020/formulas/chemistry/high-school/ejftx9d6696klvmvpql3daf7uhae7jtxhz.png)
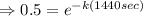
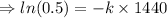
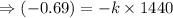
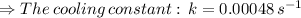
Now to find the temperature of water at time: t₂, we use the equation:
![[T(t_(2)) - T_(a)] = [T(0) - T_(a)] e^{-kt_(2)}](https://img.qammunity.org/2020/formulas/chemistry/high-school/3jxgfvsggksh2wiqviex7p2mae4q2rpt9l.png)
![\Rightarrow [T(t_(2)) - 273\, K] = [373\, K - 273\, K] e^{-(0.00048\, s^(-1))(5760\, s)}](https://img.qammunity.org/2020/formulas/chemistry/high-school/a8lfep7tvdvdqtk1wu4fli035w7nc1thqx.png)
![\Rightarrow [T(t_(2)) - 273] = [100] e^(-2.7726)](https://img.qammunity.org/2020/formulas/chemistry/high-school/uweqxk27ieqxvnbt02gm74p8wggx136q05.png)
![\Rightarrow [T(t_(2)) - 273] = 100 * 0.0625](https://img.qammunity.org/2020/formulas/chemistry/high-school/8l74ap6ygyfjnmxw22nrpvgfu7h85lvluv.png)
![\Rightarrow [T(t_(2)) - 273] = 6.25](https://img.qammunity.org/2020/formulas/chemistry/high-school/b9u1n5ysarnvk7adbs0dt29j6o2ayjoqn6.png)
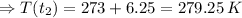
∵ 0℃ = 273 K
∴ T(t₂) = 279.25 K = 279.25 - 273 ℃ = 6.25 ℃
Therefore, the temperature of the water after 96 min (5760 s) = 279.25 K = 6.25 ℃