Answer:
Step-by-step explanation:
Given
mass of water at
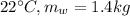
Height of water
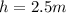
Internal Energy is defined as Energy Posses by all the molecules except macroscopic Kinetic and Potential Energy
So Potential Energy of water is used to increase the internal Energy
Potential Energy
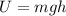
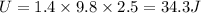
this Energy increase the internal Energy
Since Internal Energy is a function of Temperature so with increase in internal Energy will cause the temperature to rise.