Answer: The equilibrium partial pressure of sulfur dioxide, oxygen and sulfur trioxide is 0.366 atm, 0.443 atm and 0.154 atm respectively.
Step-by-step explanation:
We are given:
Initial partial pressure of sulfur dioxide = 0.52 atm
Initial partial pressure of oxygen = 0.52 atm
Initial partial pressure of sulfur trioxide = 0 atm
For the given chemical reaction:
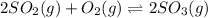
Initial: 0.52 0.52
At eqllm: 0.52-2x 0.52-x 2x
The expression of
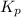 for above equation follows:
for above equation follows:
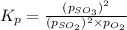
We are given:
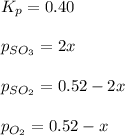
Putting values in above equation, we get:
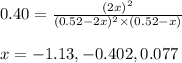
Neglecting the negative values because partial pressure cannot be negative.
So, x = 0.077
Equilibrium partial pressure of sulfur dioxide =
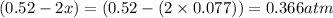
Equilibrium partial pressure of oxygen =
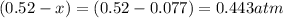
Equilibrium partial pressure of sulfur trioxide =
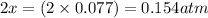
Hence, the equilibrium partial pressure of sulfur dioxide, oxygen and sulfur trioxide is 0.366 atm, 0.443 atm and 0.154 atm respectively.