Answer:
The wavelength is λ, the frequency is ν and the energy is 100E.
Step-by-step explanation:
The energy of a single photon is given by:
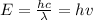
Where:
E: is the energy of a single photon,
h: is the Planck constant,
c: is the speed of light,
λ: is the photon's wavelength and
ν: is the photon's frequency
The wavelength and the frequency of the 100 photons are the same as the single-photon since the pulse of light contains 100 times the single-photon, therefore, for the pulse of light we have that the wavelength is λ, the frequency is ν, and the energy is:
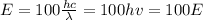
Hence, the energy of the pulse of light is 100 times the E of the single photon.
I hope it helps you!