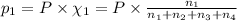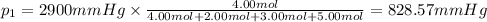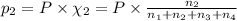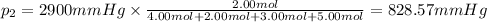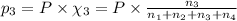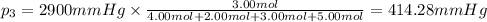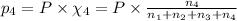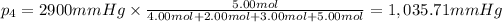Step-by-step explanation:
Moles of helium gas =
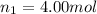
Moles of hydrogen gas =
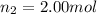
Moles of carbon dioxide gas =
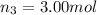
Moles of argon gas =
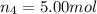
Mole fraction of helium gas =
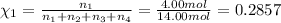
Mole fraction of hydrogen gas =
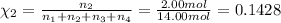
Mole fraction of carbon dioxide gas =
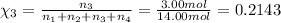
Mole fraction of argon gas =
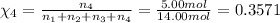
Mole percent of helium =
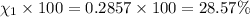
Mole percent of hydrogen=
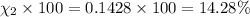
Mole percent of helium =
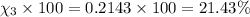
Mole percent of helium =

Total pressure of the mixture = P = 2900 mmHg
Partial pressure of helium gas =
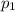
Partial pressure of hydrogen gas =
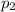
Partial pressure of carbon dioxide gas =
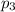
Partial pressure of argon gas =
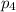
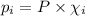
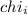 = Mole fraction of ith component
= Mole fraction of ith component