To solve this problem we will use the concept given for the exchange of heat given based on the number of moles, the specific heat and the temperature change.
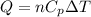
Where,
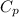 = Specific Heat
= Specific Heat
 = Change in temperature
= Change in temperature
n = Number of moles
For diatomic and polyatomic ideal gases we get: diaatomic:
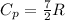
Here R is the Gas ideal Constant,
Therefore replacing we have,
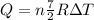
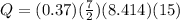
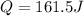
The correct answer is B.