Answer:
The pressure of the gas is 1.8 atm.
Step-by-step explanation:
Given that,
Pressure of ideal gas= 2.0 atm
rms speed of the molecule = v₀
Reduced rms speed = 0.90 v₀
We know the formula of rms speed of the ideal gas
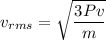
From this formula rms speed is directly proportional to square root is pressure.
We need to calculate the pressure of the gas
Using formula of rms speed
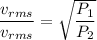
Put the value into the formula
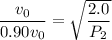
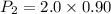
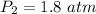
Hence, The pressure of the gas is 1.8 atm.