Answer:
1.76 g is the mass of Ne is in the container.
Step-by-step explanation:
We use the equation given by ideal gas which follows:

where,
P = pressure of the gas = 650 mm Hg
V = Volume of the gas = 2.50 L
T = Temperature of the gas =
![25^oC=[25+273]K=298K](https://img.qammunity.org/2020/formulas/physics/high-school/h3swi627jfkpg7vx7in8p5pe35bz1gwehq.png)
R = Gas constant =
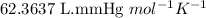
n = number of moles of Ne gas = ?
Putting values in above equation, we get:
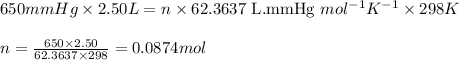
Also, molar mass of Ne = 20.1797 g/mol
So,
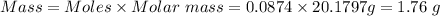
1.76 g is the mass of Ne is in the container.