Answer:
 = 7.02 ° C
= 7.02 ° C
Step-by-step explanation:
The liquid water gives heat to melt the ice (Q₁) maintaining the temperature of 0 ° C and then the two waters are equilibrated to a final temperature.
Let's start by calculating the heat needed to melt the ice
Q₁ = m L
Q₁ = 0.090 3.33 10⁵
Q₁ = 2997 10⁴ J
This is the heat needed to melt all the ice
Now let's calculate at what temperature the water reaches when it releases this heat
Q = M
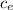 (T₀ -
(T₀ -
 )
)
Q₁ = Q
 = T₀ - Q₁ / M
= T₀ - Q₁ / M
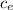
 = 20.0 - 2997 104 / (0.600 4186)
= 20.0 - 2997 104 / (0.600 4186)
 = 20.0 - 11.93
= 20.0 - 11.93
 = 8.07 ° C
= 8.07 ° C
This is the temperature of the water when all the ice is melted
Now the two bodies of water exchange heat until they reach an equilibrium temperature
Temperatures are
Water of greater mass T₀₂ = 8.07ºC
Melted ice T₀₁ = 0ºC
M
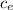 (T₀₂ -
(T₀₂ -
 ) = m
) = m
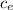 (
(
 - T₀₁)
- T₀₁)
M T₀₂ + m T₀₁ = m
 + M
+ M

 = (M T₀₂ + 0) / (m + M)
= (M T₀₂ + 0) / (m + M)
 = M / (m + M) T₀₂
= M / (m + M) T₀₂
let's calculate
 = 0.600 / (0.600 + 0.090) 8.07
= 0.600 / (0.600 + 0.090) 8.07
 = 7.02 ° C
= 7.02 ° C