Answer:
65.6%
Step-by-step explanation:
Let's consider the following reaction.
3 Cl₂ + 6 KOH → KClO₃ + 5 KCl + 3 H₂O
We can establish the following relations.
- The molar mass of Cl₂ is 70.9g/mol.
- The molar ratio of Cl₂ to KCl is 3:5.
- The molar mass of KCl is 74.6 g/mol.
The theoretical yield of KCl from 30.0 g of Cl₂ is:
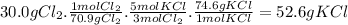
The real yield of KCl is 34.5 g. The percent yield is:
(34.5 g / 52.6 g) × 100% = 65.6%