Answer:
atomic concentration = 2 atoms/unit cell
lattice parameter: a= 3.22 x 10⁻¹⁰ m
atomic radius: r= 1.39 x 10⁻¹⁰m
Step-by-step explanation:
The atomic concentration is the number of atoms that can fit into a unit cell. It is a known number for each unit cell crystal structure. For a BCC (body-centered cube) crystal structure, atomic concentration is 2 atoms/unit cell because there are a 1/8 part of an atom in each corner of the cube (1/8 x 8= 1 atom) and 1 central atom in the central position of the cube ⇒ n= 1 atom + 1 atom= 2 atoms/unit cell
In order to calculate the lattice parameter a, we introduce the atomic mass 95.94 g/mol and the density 10.22 g/cm³ in the expression for the volume of the cube:
Vc= a³=
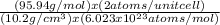
a³= 3.12 x 10⁻²³ m³
⇒ a = ∛(3.12 x 10⁻²³ m³) = 3.22 x 10⁻¹⁰m
Once we know the lattice parameter a, we can calculate the atomic radius r by using the expression of a for a BCC structure:
a=
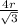
⇒ r= a x √3/4= (3.22 x 10⁻¹⁰ m) x √3/4 = 1.39 x 10⁻¹⁰ m