The given question is incomplete. The complete question is as follows.
When 70.4 g of benzamide (
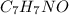 ) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is
) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is
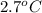 lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (
lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (
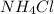 ) are dissolved in the same mass of X, the freezing point of the solution is
) are dissolved in the same mass of X, the freezing point of the solution is
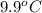 lower than the freezing point of pure X.
lower than the freezing point of pure X.
Calculate the Van't Hoff factor for ammonium chloride in X.
Step-by-step explanation:
First, we will calculate the moles of benzamide as follows.
Moles of benzamide =
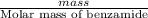
=
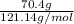
= 0.58 mol
Now, we will calculate the molality as follows.
Molality =
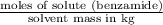
=
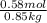
= 0.6837
It is known that relation between change in temperature, Van't Hoff factor and molality is as follows.
dT =
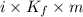 ,
,
where, dT = change in freezing point =
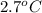
i = van't Hoff factor = 1 for non dissociable solutes
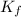 = freezing point constant of solvent
= freezing point constant of solvent
m = 0.6837
Therefore, putting the given values into the above formula as follows.
dT =
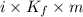 ,
,
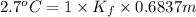
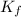 = 3.949 C/m
= 3.949 C/m
Now, we use this
 value for calculating i for
value for calculating i for
So, moles of ammonium chloride are calculated as follows.
Moles of
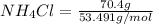
= 1.316 mol
Hence, calculate the molality as follows.
Molality =
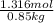
= 1.5484
It is given that value of change in temperature (dT) =
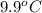 . Thus, calculate the value of Van't Hoff factor as follows.
. Thus, calculate the value of Van't Hoff factor as follows.
dT =
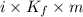
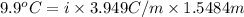
i = 1.62
Thus, we can conclude that the value of van't Hoff factor for ammonium chloride is 1.62.