Answer: The IUPAC name of the carboxylic acid formed is ethanoic acid.
Step-by-step explanation:
The basic rules for naming of organic compounds are :
- First select the longest possible carbon chain.
- The longest possible carbon chain should include the carbons of double or triple bonds.
- The naming of alkane is done by adding the suffix -ane, alkene by adding the suffix -ene, alkyne by adding the suffix -yne and carboxylic acid by adding the suffix -oic acid.
- The numbering is done in such a way that first carbon of double or triple bond gets the lowest number.
- The carbon atoms of the double or triple bond get the preference over the other substituents present in the parent chain.
For the given chemical reaction, the equation follows:
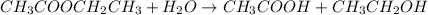
When hydrolysis of ester takes place, it leads to the formation of an alcohol and a carboxylic acid.
So, when hydrolysis of ethyl ethanoate occurs, it produces ethanol and a 2- Carbon carboxylic acid named as ethanoic acid.
Hence, the IUPAC name of the carboxylic acid formed is ethanoic acid.