Step-by-step explanation:
Reaction equation for the given chemical reaction is as follows.
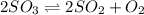
Equation for reaction quotient is as follows.
Q =
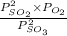
=
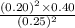
= 0.256
As, Q > K (= 0.12)
The effect on the partial pressure of
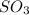 as equilibrium is achieved by using Q, is as follows.
as equilibrium is achieved by using Q, is as follows.
- This means that there are too much products.
- Equilibrium will shift to the left towards reactants.
- More
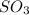 is formed.
is formed.
- Partial pressure of
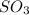 increases.
increases.