Answer:
a.
The higher quantum number is n+1 = 12 +1 =13
b.
The higher quantum number is n = 12
c.
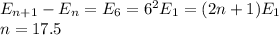
this is not integer so impossible to find pair of adjacent level
Step-by-step explanation:
Let the quantum number pair will be n and n+1
equation of energy of particle in one dimensional box is =
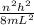
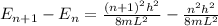
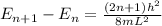
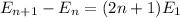
Which leads to
2n+ 1 =25
n= 12
a.
The higher quantum number is n+1 = 12 +1 =13
b.
The higher quantum number is n = 12
c.
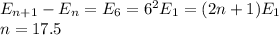
this is not integer so impossible to find pair of adjacent level