Answer:
the sample undergo
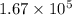 disintegrations per second.
disintegrations per second.
Step-by-step explanation:
Given:
The amount of sulphur purchased is 0.671 μCi
To find:
disintegrations per second = ?
Solution:
Some of the conversions are
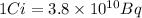
1 rad = 0.01 Gy
1Gy = 1 j/kg tissue
1 rem = 0.01 Sv
1Sv = 1 j/Kg
Using these conversions,
The decay rate of this sample is calculated as
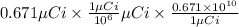
=
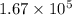 disintegrations per second
disintegrations per second