Answer:
Major product is Hept-1-en-4-yne
Step-by-step explanation:
Sodium amide (
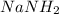 ) gives acid-base reaction with terminal alkyne preferably than terminl alkene. Because terminal alkyne are more acidic due to sp-hybridization of carbon atom.
) gives acid-base reaction with terminal alkyne preferably than terminl alkene. Because terminal alkyne are more acidic due to sp-hybridization of carbon atom.
In the first step, sodium amide first deprotonates pent-1-en-4-yne to produce an alkenylide anion.
In the second step, the alkenylide anion gives a
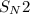 reaction with bromoethane to produce hept-1-en-4-yne.
reaction with bromoethane to produce hept-1-en-4-yne.
Full reaction scheme has been shown below.